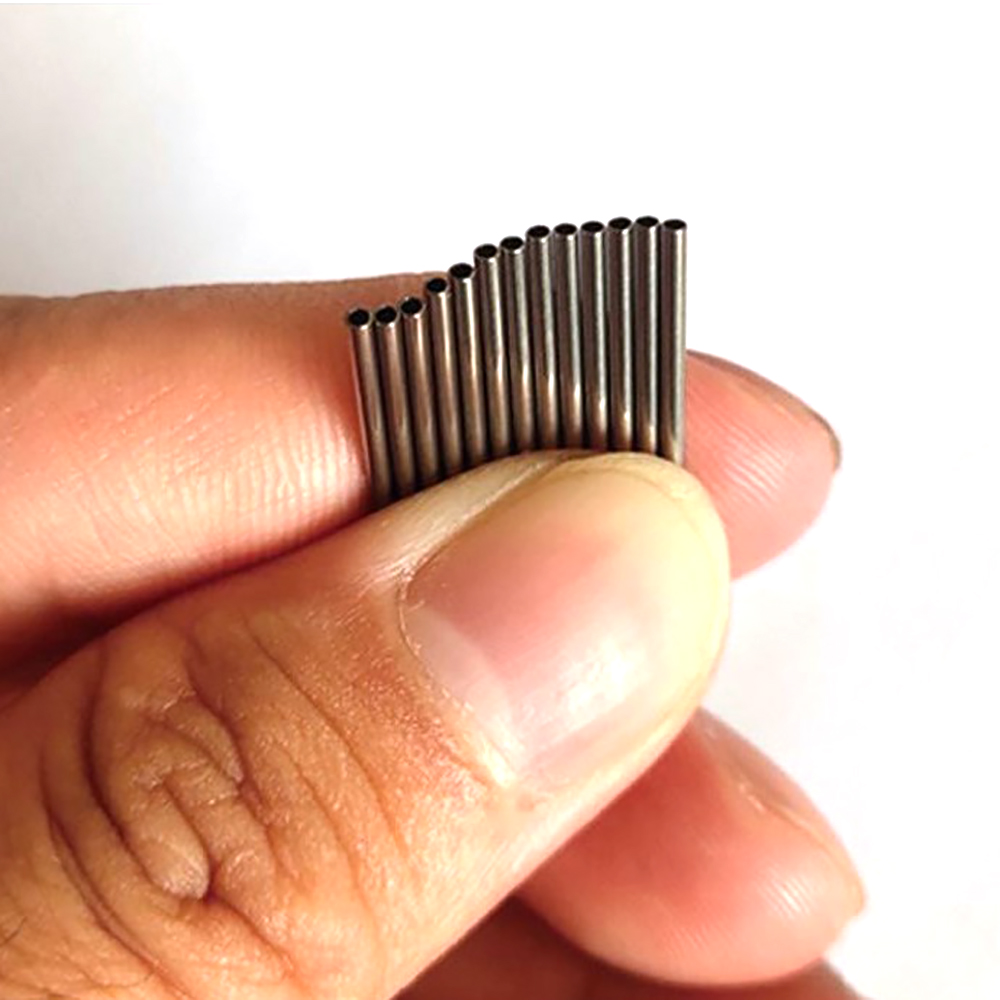
At our company, we’re all about crafting ISO 5832-1 compliant 316LVM stainless steel tube for medical use that powers life-changing procedures, from heart stents to bone implants. These tubes aren’t just metal—they’re precision-engineered components that surgeons trust for reliability and biocompatibility in the toughest medical applications. With a focus on high purity and performance, we’re delivering solutions that make healthcare safer and more effective.
Let’s break down what makes our 316LVM stainless steel tube for medical use a standout. This vacuum-melted alloy is the gold standard, with 17-19% chromium for a tough corrosion barrier, 13-15% nickel for durability, and 2.5-3.5% molybdenum to fight pitting in bodily fluids. With carbon kept below 0.03%, it’s weldable and meets ISO 5832-1 and ASTM F138 standards for implant-grade safety. We produce these tubes in diameters from 0.3mm to 12mm, with walls as thin as 0.05mm, perfect for everything from micro-stents to robust bone nails. Our advanced annealing boosts tensile strength to 860-1000 MPa, ensuring implants and devices can handle intense biomechanical stresses.
For more details, pls directly contact us.
We’re obsessed with precision. Our tubes are cold-drawn to tolerances of ±0.005mm, ensuring seamless integration into complex devices like catheters or spinal rods. Electropolishing creates a Ra <0.15µm finish, minimizing bacterial adhesion and thrombus formation, which is critical for blood-contact applications. We offer bioactive coatings, like hydroxyapatite, to enhance bone integration, and our fatigue testing simulates years of stress—think millions of heartbeats or steps—to prove durability. Very low magnetic permeability keeps our tubes MRI-safe, a must for post-op diagnostics.
The medical device market is thriving, valued at USD 492.3 billion in 2025 and projected to hit USD 747.1 billion by 2032 at a 5.8% CAGR. Aging populations are a huge driver—by 2030, over 1.4 billion people will be over 60, spiking demand for implants and minimally invasive devices. Cardiovascular diseases affect over 900 million people globally, and trauma cases, like fractures from accidents, are climbing, with over 4 million annually in the U.S. alone. Minimally invasive techniques, like stenting and percutaneous fixation, are cutting recovery times by up to 50%, and our ISO 5832-1 compliant 316LVM stainless steel tube for medical use is built for this, offering strength and precision for these advanced procedures.
Comparison of Medical Stainless Steel Grades, Materials, and Applications
Grade | Composition | Key Properties | Corrosion Resistance | Biocompatibility | Applications | Advantages | Limitations |
|---|---|---|---|---|---|---|---|
316L | Fe (60-70%), Cr (16-18%), Ni (10-14%), Mo (2-3%), C (<0.03%) | Tensile: 485-620 MPa, Yield: 170-290 MPa, Elongation: 40-50%, Hardness: 95 HRB | Excellent (passive oxide layer, resists pitting) | High, minimal ion release, rare Ni sensitivity | Bone plates, screws, stents, hip stems, dental implants | Cost-effective, machinable, fatigue-resistant | Possible Ni sensitivity, heavier than Ti |
304L | Fe (65-74%), Cr (18-20%), Ni (8-10.5%), C (<0.03%) | Tensile: 485-550 MPa, Yield: 170-240 MPa, Elongation: 40-55%, Hardness: 92 HRB | Good, less resistant to pitting than 316L | Moderate, higher Ni release risk | Temporary implants, surgical tools, guidewires | Affordable, easy to form, widely available | Limited for long-term implants due to corrosion |
17-4 PH | Fe (70-78%), Cr (15-17.5%), Ni (3-5%), Cu (3-5%), C (<0.07%) | Tensile: 930-1100 MPa, Yield: 725-860 MPa, Hardness: 30-44 HRC | Very good, but less than 316L in saline | Good, but less biocompatible than 316L | Load-bearing implants, surgical instruments | High strength, heat-treatable, durable | Complex processing, less corrosion-resistant |
420 | Fe (80-90%), Cr (12-14%), C (0.15-0.4%) | Tensile: 700-950 MPa, Yield: 340-450 MPa, Hardness: 45-50 HRC | Moderate, prone to pitting in body fluids | Moderate, not ideal for long-term implants | Cutting tools, temporary pins, dental drills | High hardness, wear-resistant, sharpenable | Poor corrosion resistance for permanent use |
440C | Fe (78-85%), Cr (16-18%), C (0.95-1.2%) | Tensile: 760-1000 MPa, Yield: 450-600 MPa, Hardness: 56-60 HRC | Moderate, better than 420 but less than 316L | Limited, high carbon affects biocompatibility | Surgical blades, high-wear tools | Extremely hard, excellent edge retention | Not suitable for long-term implants |
F138 (316LVM) | Fe (60-70%), Cr (17-19%), Ni (13-15%), Mo (2.25-3.5%), C (<0.03%) | Tensile: 490-690 MPa, Yield: 190-300 MPa, Elongation: 40-50%, Hardness: 95 HRB | Superior, optimized for medical use | Excellent, lowest ion release, vacuum-melted | Orthopedic implants, cardiovascular stents | Enhanced purity, top biocompatibility | Higher cost than standard 316L |
303 | Fe (65-75%), Cr (17-19%), Ni (8-10%), S (0.15-0.35%) | Tensile: 500-620 MPa, Yield: 240-290 MPa, Elongation: 35-50%, Hardness: 90 HRB | Moderate, sulfur reduces corrosion resistance | Moderate, not ideal for permanent implants | Machined components, non-implant devices | Excellent machinability, cost-effective | Not suitable for long-term implants |
Nitronic 60 | Fe (60-70%), Cr (16-18%), Ni (8-9%), Mn (7-9%), N (0.08-0.18%) | Tensile: 620-793 MPa, Yield: 345-414 MPa, Hardness: 95-100 HRB | Very good, resists galling and wear | Good, but less studied for implants | Wear-resistant implants, joint components | High wear resistance, galling resistance | Limited medical use, higher cost |
For more details, pls directly contact us

Regulatory standards are getting stricter, with the FDA and EU MDR demanding robust biocompatibility and traceability. Our 316LVM tubes exceed ISO 10993 standards for long-term implantation, with minimal ion release to prevent tissue reactions. Sustainability is a growing focus—hospitals want recyclable materials, and stainless steel’s near-100% recyclability makes it a green choice. The medical tubing market is set to grow from USD 812.59 million in 2025 to USD 1,547.53 million by 2034 at a 6.7% CAGR, with high-purity alloys like 316LVM leading for their reliability in critical applications.
Emerging trends are shaping the future. Bioactive coatings are gaining traction to improve bone integration and reduce clotting risks in vascular devices. Robotic-assisted surgeries, projected to hit USD 14.4 billion by 2026, demand ultra-precise tubing for articulated systems, where 316LVM excels. The rise of outpatient clinics—growing at 6.8% CAGR—calls for cost-effective, durable components, and our tubes deliver without breaking the bank. Personalized medicine is another frontier, with 3D-printed implants driving demand for customizable tubes to match patient anatomies.
Applications for our ISO 5832-1 compliant 316LVM stainless steel tube for medical use are vast. In orthopedics, they form intramedullary nails and cannulated screws for long bones like the femur, stabilizing fractures for faster healing. For spinal surgeries, they’re used in rods and pedicle screws, supporting vertebrae under heavy loads. In vascular applications, our tubes are the backbone of coronary and peripheral stents, ensuring reliable expansion to restore blood flow. They’re also key in guidewires and catheters, navigating tortuous vessels for angioplasty or diagnostic procedures.
Minimally invasive procedures are a sweet spot. Our thin-walled tubes enable percutaneous delivery of implants, reducing incision sizes and hospital stays. In neurovascular applications, they support stents for aneurysm treatment, offering precision for delicate brain vessels. Even in dental implants, our tubes serve as posts for crowns, balancing strength with biocompatibility. The orthopedic implants market, valued at USD 46.1 billion in 2025, is growing at 5.2% CAGR to 2032, while the stent market is set to hit USD 17.40 billion by 2034 at a 6% CAGR, underscoring the demand for versatile, high-purity tubing.
When it comes to standing out, our company’s approach to ISO 5832-1 compliant 316LVM stainless steel tube for medical use is unmatched. While standard suppliers might offer generic tubing, we customize with tailored annealing and laser-cutting to optimize performance for specific devices, like high-load spinal implants or intricate stents. Our in-house testing is rigorous—corrosion trials in simulated body fluids and finite element analysis predict behavior under stress, ensuring zero failures. This reliability means fewer complications, saving hospitals and patients from costly revisions.
We’re lean on production, using automated cold-rolling to achieve tolerances of ±0.005mm, perfect for micro-tubes in guidewires or stents. Cost-wise, we keep it competitive by streamlining material use, delivering implant-grade quality at prices that work for manufacturers. Surgeons praise our tubes’ consistency, ensuring predictable performance in high-stakes procedures like femoral nailing or coronary interventions. Unlike others who might skip advanced surface treatments, we electropolish and apply coatings to boost biocompatibility, reducing healing times by up to 25%.
Sustainability is core to our ethos—we recycle 95% of scrap metal, aligning with healthcare’s green push. Our supply chain is rock-solid, with just-in-time delivery to keep production lines running, even during global disruptions. We also offer design collaboration, working with manufacturers to tweak tube specs for next-gen devices, a service most suppliers can’t match. Clients highlight our fast prototyping—often under two weeks—as a game-changer for innovative designs.
Comparison Parameters Table
| Parameter | 316LVM Stainless Steel | 316L Stainless Steel | 304 Stainless Steel | Titanium Alloy (e.g., Ti-6Al-4V) |
|---|---|---|---|---|
| Carbon Content (%) | ≤0.03 | ≤0.03 | ≤0.08 | N/A (No carbon) |
| Corrosion Resistance | Superior (vacuum-melted, resists body fluids) | Excellent (Mo, good for implants) | Good (Cr, less in chlorides) | Outstanding (oxide layer, best for implants) |
| Tensile Strength (MPa) | 860-1000 | 485-620 | 515-690 | 860-950 |
| Yield Strength (MPa) | 690-860 | 170-310 | 205-310 | 760-830 |
| Biocompatibility | Exceptional (high purity, low inclusions) | High (implant-grade) | Good (short-term use) | Excellent (bone integration, lightweight) |
| Density (g/cm³) | 8.0 | 8.0 | 8.0 | 4.5 |
| Magnetic Properties | Very low permeability | Non-magnetic | Non-magnetic | Non-magnetic |
| Cost Effectiveness | Premium for purity | Affordable | Most affordable | High, for lightweight needs |
| Common Medical Use | Stents, orthopedic implants | Stents, bone screws | Surgical instruments | Orthopedic implants, lightweight fixators |
Looking ahead, we’re diving into smart medical devices, exploring 316LVM tubes with embedded sensors for real-time monitoring of implant performance, tapping into IoT trends. With personalized medicine growing—custom implants are expected to hit USD 9 billion by 2030—our flexible production is ready to deliver patient-specific solutions. The rise of robotic-assisted surgeries and AI-driven design means our tubes must support precision systems, and we’re optimizing for that now.
In essence, choosing our ISO 5832-1 compliant 316LVM stainless steel tube for medical use means partnering with a team obsessed with quality and innovation. We’re not just making tubes; we’re enabling life-changing procedures. From high-purity materials to tailored designs, we’re built to lead in a market craving reliability and precision. As medical tech evolves with demographics and innovation, our 316LVM tubes remain a trusted cornerstone, ready for tomorrow’s challenges.
To sum it up, our strengths shine: exceptional biocompatibility, customizable precision, and a green approach that sets us apart. Whether it’s a routine fracture repair or a complex vascular stent, our ISO 5832-1 compliant 316LVM stainless steel tube for medical use delivers the performance doctors need to transform lives.
For more details, pls directly contact us.




About Us:
Our 12,000㎡ factory is equipped with complete capabilities for research, production, testing, and packaging. We strictly adhere to ISO 9001 standards in our production processes, with an annual output of 1,200 tons. This ensures that we meet both quantity and quality demands. Furthermore, all products undergo rigorous simulated environment testing including high temperature, high pressure, and corrosion tests before being dispatched, ensuring they meet customer specifications.
For all our clients, we offer timely and multilingual after-sales support and technical consulting, helping you resolve any issues swiftly and efficiently.

Client Visits
Building Stronger Partnerships

We support all kinds of testing:


FAQs:
Why is ISO 5832-1 compliant 316LVM stainless steel ideal for medical use?
It offers exceptional corrosion resistance, high purity, and biocompatibility, making it perfect for medical implants and devices like stents, bone screws, and catheters that require long-term reliability in the body.What is the material composition of 316LVM stainless steel for medical applications?
It contains 17-19% chromium, 13-15% nickel, 2.5-3.5% molybdenum, and carbon below 0.03%, vacuum-melted for purity, ensuring corrosion resistance and compliance with ISO 5832-1 and ASTM F138 standards.What are the primary applications of 316LVM stainless steel tubes in medical devices?
These tubes are used in vascular stents, orthopedic implants, guidewires, and surgical needles, providing structural support and precision for minimally invasive and load-bearing applications.How do industry trends influence the use of 316LVM in medical devices?
The medical device market, valued at USD 492.3 billion in 2025, is projected to grow at a 5.8% CAGR to USD 747.1 billion by 2032, driven by demand for biocompatible, high-purity materials in minimally invasive procedures.What mechanical properties make 316LVM suitable for medical tubing?
It provides tensile strength of 860-1000 MPa, yield strength of 690-860 MPa, elongation over 40%, and high fatigue resistance, ensuring durability in dynamic medical applications like implants and catheters.Is 316LVM stainless steel biocompatible for medical use?
Yes, it meets ISO 10993 and ISO 5832-1 standards, with minimal ion release and low tissue reactivity, ideal for long-term implants and blood-contact devices.What emerging trends support 316LVM in medical applications?
Trends include bioactive coatings, growth in robotic-assisted surgeries, and a medical tubing market expected to reach USD 1,547.53 million by 2034 at a 6.7% CAGR, favoring high-purity alloys for advanced devices.How does 316LVM handle sterilization for medical applications?
It withstands autoclaving, gamma irradiation, and chemical sterilization without corrosion, maintaining surface integrity and biocompatibility for sterile medical environments.